

The European Chemical Society (EuChemS) has designed this table to make people reflect (and hopefully act) on the issue of element scarcity. Well, this one is clearly based on the most traditional arrangement, but it is actually giving out completely different information. (Ch1902/Wikimedia/CC-BY-SA-3.0) The Periodic Table of Element Scarcity (2018)
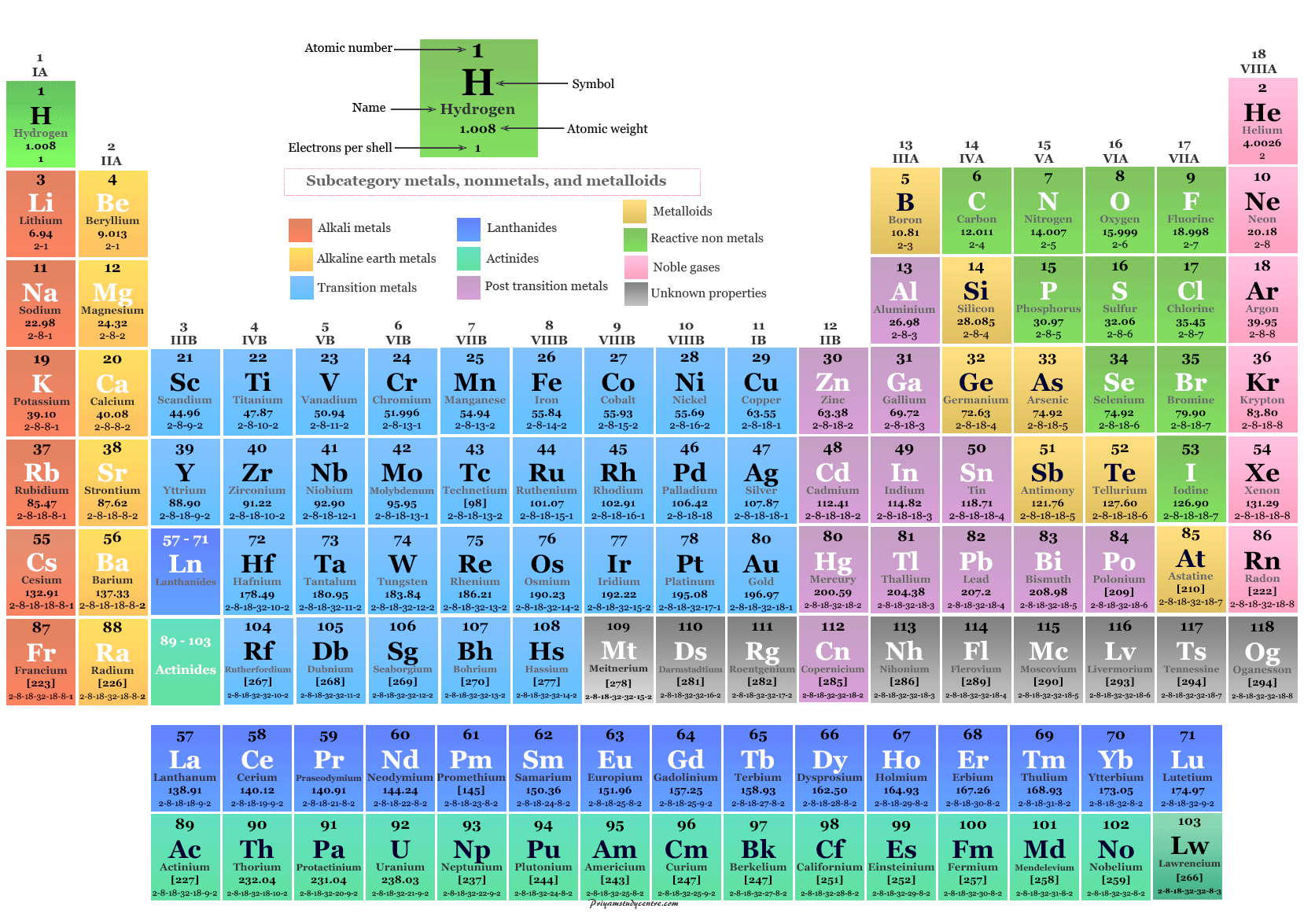
The table is so neat that elements with similar properties appear grouped together in blocks or connected by solid arrows (see for example the inert gases on the left). This irregular spiral is a systematic arrangement of the natural elements (further extended to element 104) by their number of electrons. The Race-Track Periodic Table (1933)Ĭhemist and sci-fi author John Clark created a race-track-like table in 1933 (left), whose nicely colored version became popular after LIFE magazine’s issue on The Atom in 1945 (right). We will now show you some of our favorite alternative arrangements. Therefore, the standard periodic table does not represent the actual linearity of the elemental properties, hence the efforts some have put into devising other (more accurate) forms of the same element order. This was done so that the middle rows would clearly show a period of 18, in detriment of the period of 8 occurring before it and that of 32 occurring after it.
Moreover, obvious gaps have been inserted at the top of the table that is, H/He, Be/B, and Mg/Al should be closer in space.
#Periodic table f series#
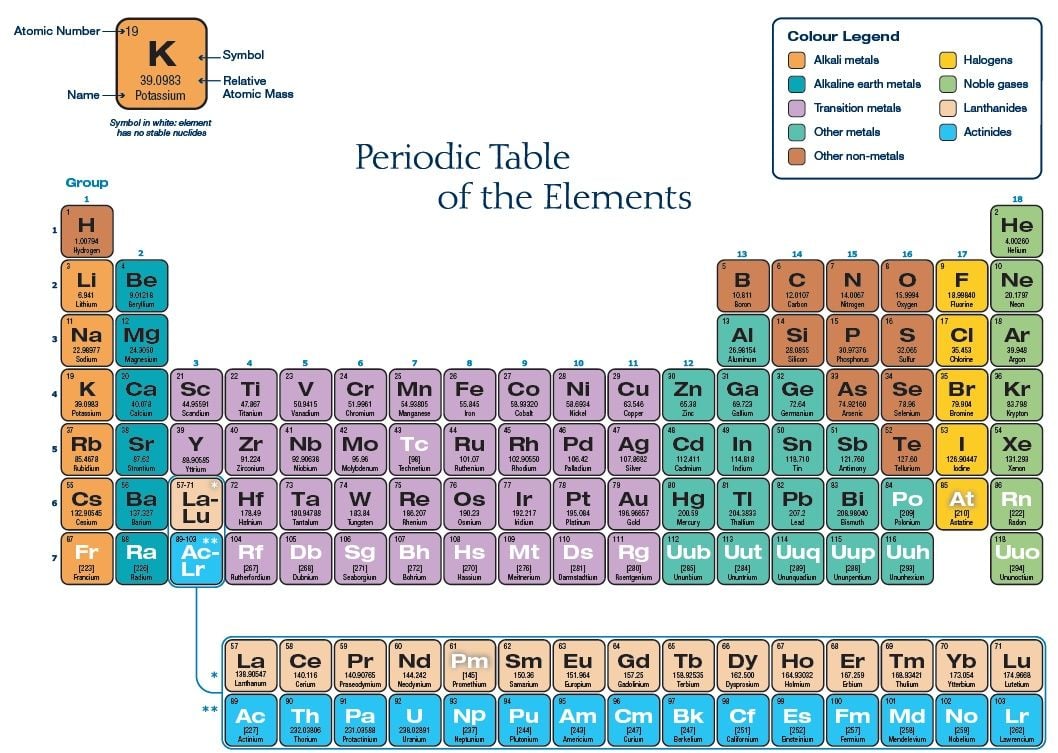
The truth is that Mendeleev’s periodic table is artificially split into rows instead of being a continuum, even though the series of elements is in fact linear. Following our brief introduction to the history of the Periodic Law and its translation into a table, we would like to remind you now that elements and their relationships can be showcased in many different ways.


 0 kommentar(er)
0 kommentar(er)
